Chemistry
Chemistry
Water is a very good solvent. In fact, pure water is seldom found in nature because it readily dissolves the many chemicals that wash off
the land or pour out of effluent pipes. Most Canadians use surface water from ponds, lakes or rivers for drinking and household use. Clean water is so important to healthy communities. Measuring the concentration of a few key chemicals can help indicate river water quality.
Is your river healthy?
Dissolved Oxygen
Oxygen is an element essential to all living things. Dissolved oxygen is perhaps the most important abiotic or non-living factor affecting aquatic communities such as rivers. Fish and many macroinvertebrates nymphs and larvae are equipped with gills to extract oxygen from the water they live in.
Pre-packaged chemistry test kits are used to measure the concentration of dissolved oxygen. The amount of oxygen dissolving in water is affected by temperature, mixing, decay and pollution. Low levels of dissolved oxygen are harmful to many species and can indicate that pollution has entered the water.
Understanding the basic science of dissolved oxygen will help you interpret the data collected from your local river. Take a deep breath and lick below to learn more about dissolved oxygen!
Background Information
The air that we breathe every second of every day is composed of 21% molecular oxygen gas (O2). This means that about one out of every five molecules in the atmosphere is an oxygen molecule (particle).
Approximately 75% of the atmospheric oxygen is produced by one-celled plants (phytoplankton) floating in the oceans. Oxygen is required by all living things, as well as the processes of combustion (burning) and oxidation (rusting of iron).
What is Dissolved Oxygen?
Dissolved oxygen is merely the oxygen molecules that have mixed in with water molecules. It gets there by diffusing from the air; when trapped by aeration or bubbling; and as a waste product from green plant photosynthesis.
Oxygen gas dissolves in water much like the carbon dioxide responsible for the fizz in a can of soda pop. Carbon dioxide (CO2), however, is 200 times more soluble in water than oxygen.
Oxygen is not very soluble and occurs dissolved in only trace amounts. The tiny amounts of dissolved oxygen are measured in the range of 1-14 milligrams per litre (mg/L). While one out of every five molecules in the atmosphere is oxygen, in water, only 1-14 molecules out of a million are oxygen. Said another way, the concentration of dissolved oxygen is 1-14 parts per million (ppm).
Note: The concentration units of mg/L and ppm are equivalent. (mg/L = ppm)
Aquatic organisms such as fish and macroinvertebrates rely on gills for breathing dissolved oxygen. Bacteria also use oxygen when they decompose dead organisms. So far, however, no one has devised a way for humans to extract oxygen from water during a dive.
What Natural Processes Increase Dissolved Oxygen Levels?
Oxygen is only slightly soluble. Several abiotic (non-living) and biotic (living) processes help to increase the amount of dissolved oxygen.
Abiotic Factors That Increase Dissolved Oxygen
- Faster moving water holds more oxygen than slow or standing water. Turbulence or splashing whitewater falling over weirs, dams and rocks traps air into the water, increasing dissolved oxygen levels.
- The mixing of water from top to bottom in a river ensures that oxygen levels are fairly constant no matter what the depth.
- Water temperature can affect dissolved oxygen levels. Cold water is capable of holding more oxygen than warm water.
- Increasing barometric pressure can force more oxygen into solution. This effect is also accomplished with decreasing altitude. Water at lower elevations is under greater pressure and contains more dissolved oxygen.
Biotic Factors That Increase Dissolved Oxygen
- Photosynthesis by aquatic plants and algae adds oxygen into water during daylight. Bubbles of oxygen gas can actually be seen clinging to and rising from submerged leaves under direct sunlight.
During photosynthesis, plants use sunlight in a reaction with carbon dioxide and water. The result is the production of glucose (sugar) and the release of oxygen gas.
6CO2(g) + 6H2O(l) + sunlight --> C6H12O6(s) + 6O2(g)
What Natural Processes Decrease Dissolved Oxygen Levels?
Oxygen is only slightly soluble at the best of times. Compounding this problem are several abiotic (non-living) and biotic (living) processes that actually decrease the amount of dissolved oxygen.
Abiotic Factors That Decrease Dissolved Oxygen
- Warm water holds less dissolved oxygen than cold water. (This same rule also applies when warm soda pop fizzes out of a can or glass. The carbon dioxide dissolved in the liquid is less soluble at warmer temperatures.)
- Shallow water holds less dissolved oxygen. River levels tend to drop throughout the summer as mountain snow finishes melting. Shallow water moves more slowly and heats up faster. This often creates a critical time for aquatic organisms as dissolved oxygen levels drop.
- Turbid or cloudy water may have lower oxygen levels. Less sunlight is able to penetrate the water and less photosynthesis is likely to occur.
- Decreasing barometric pressure can release oxygen out of a solution. This effect is also accomplished with increasing altitude. Water at higher elevations is under less pressure and contains less dissolved oxygen.
Biotic Factors That Decrease Dissolved Oxygen
- Levels of dissolved oxygen decrease during the night because photosynthesis stops. A daily graph of oxygen concentrations shows an undulating or wavy pattern with the lowest readings occurring just before dawn on hot summer mornings.
- With no photosynthesis occurring at night, animal and plant respiration gradually reduces the
C6H12O6(s) + 6O2(g) + enzymes --> 6CO2(g) + 6H2O(l) + energy
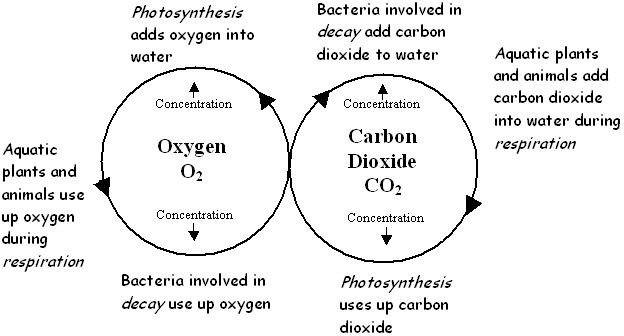
How is Oxygen Cycled Through the Biosphere?
There are two main processes occurring in all ecosystems - energy flow and material cycling. Oxygen and carbon dioxide are cycled in a symbiotic relationship (mutualism) between plants, animals and bacteria. This carbon-oxygen cycle operates in water in much the same way as it does on land:
- Green plants use carbon dioxide and release oxygen during photosynthesis.
- Animals use oxygen and release carbon dioxide during respiration.
- Bacteria use oxygen and release carbon dioxide during decomposition.
Three natural processes cause the cycling of oxygen and carbon dioxide in aquatic ecosystems:
- photosynthesis
- cellular respiration
- decomposition
The Effects of Low Dissolved Oxygen Levels
High levels of dissolved oxygen are necessary to maintain diversity in aquatic communities. High dissolved oxygen levels can even make drinking water taste better. It's therefore important to understand the human activities that might cause reduced dissolved oxygen levels.
What Human Activities Cause Low Levels of Dissolved Oxygen?
Several human activities can affect the oxygen concentrations in a river:
- Warm water discharged from factories, wastewater treatment plants or power plants reduces dissolved oxygen levels. This is known as thermal pollution.
- Warm water with low oxygen levels is found in slow, shallow rivers created by withdrawing water for irrigation or the filling of reservoirs. In 2000, there was concern that irrigation water taken from the Little Bow River might leave fish in a desperate situation during late summer.
- Warm water with low oxygen levels can result when vegetation is removed from stream banks during landscaping, logging or clearing farmland. Less vegetation results in less shade from the sun and higher water temperatures result.
- Nutrients added to water by fertilizers washing-off fields or added by urban sewage can produce excessive plant or algae growth in rivers. During late summer or winter, decomposing bacteria break down the masses of dying aquatic plants and algae. This decay process consumes large amounts of dissolved oxygen, affecting fish and other pollution sensitive organisms.
- Animal and plant waste (pulp, manure, vegetable peels, blood, leaves, grass) entering rivers from pulp mills, feedlots, dairies, food-processing plants, meatpacking plants, forests and lawns create eutrophic or organically enriched conditions. This organic loading may result in low oxygen levels as bacteria decompose the material. When populations of microscopic decomposers rapidly increase, a situation of high biological oxygen demand (B.O.D.) is created. Under extreme conditions of high B.O.D., anaerobic bacteria produce hydrogen sulfide gas with a rotten egg smell.
How do low oxygen levels affect aquatic ecosystems?
Dissolved oxygen levels affect the survival of aquatic organisms. Trout and the nymphs of mayflies or stoneflies are found only in water with high oxygen concentrations. If dissolved oxygen levels are low, only organisms such as leeches, snails and roundworms can survive. Fish and stonefly nymphs die while trapped in water with decreased oxygen levels. Low levels of dissolved oxygen can result in significant fish kills, especially in late summer or during the winter.
Dissolved Oxygen (DO) Test Kits
Dissolved oxygen levels can be tested with portable chemistry kits. Using the kits require several steps and careful attention to instructions.
Contents of Dissolved Oxygen Test Kits
WARNING: The chemicals in this kit may be hazardous to the health and safety of the user if inappropriately handled.
- Read all labels
- Wear gloves and goggles
- Collect the waste solution in a plastic Nalgene waste bottle. This solution should be neutralized with sodium thiosulfate before flushing into the sanitary sewer system.
Instructions for Using the Dissolved Oxygen Test Kit
WARNING: The chemicals in this kit may be hazardous to the health and safety of the user if inappropriately handled. Please read all warnings carefully before performing the test and use appropriate safety equipment.
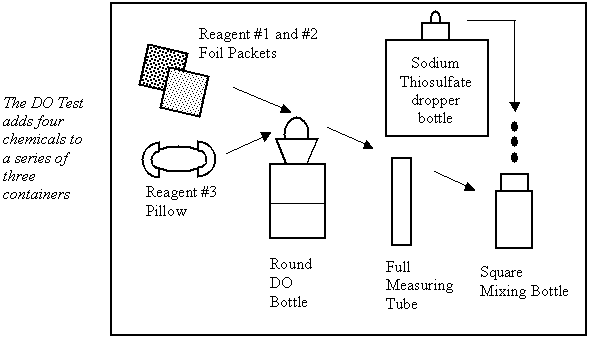
1. Place the kit in a safe, dry place on the ground. Look for a water sample collection site upstream of other classmates in the river.
2. Use the Dissolved Oxygen (DO) Bottle to collect flowing river water. Slowly submerge the tilted bottle with the opening pointing downstream.
3. Fill the DO bottle to 1/2 way up the neck of the bottle. Do not stopper the bottle yet. Stand the bottle back in the test kit.
4. Wear safety goggles and gloves. Open a Dissolved Oxygen #1 Foil Packet and a Dissolved Oxygen #2 Foil Packet. Carefully tap the contents of each packet into the DO bottle. Discard the empty packets into the garbage bag provided. This solution should be rinsed off if skin contact occurs.
5. Place the glass stopper into the DO bottle. Press on the stopper and quickly tip any overflow solution from the top of the DO bottle into the DO Waste Bottle. If an air bubble is trapped under the stopper, ask your guide for help.
6. Grip the bottle and stopper firmly. Invert to mix. A brownish flocculent (floc) precipitate will form. If any powdered reagent is left stuck to the bottom of the bottle it will not affect the test results.
7. Place the DO bottle back in the test kit. Allow the sample to stand until the floc settles to the white line on the bottle. Invert the bottle again and let the floc settle a second time (4 to 5 minutes).
8. Use the clippers to open one Dissolved Oxygen #3 Powder Pillow. Carefully add the acid contents of the pillow to the DO bottle. Restopper the bottle and tip any overflow into the DO Liquid Waste Bottle. At this point, trapped air bubbles are not important. Invert to mix. The floc will dissolve and a yellow colour will appear if oxygen is present.
9. Work over top of the open waste bottle while completely filling the plastic measuring tube with the prepared DO sample. Use the upside down square mixing bottle as a lid, then quickly flip both containers to transfer the contents from the tube into the square mixing bottle.
10. Add Sodium Thiosulfate drop-by-drop into the square mixing bottle until the solution changes from yellow to colourless. Hold the dropper straight up and make sure that drops fall directly into the sample liquid. Swirl to mix after each drop and compare against a white background. Count each drop and record the total number added. Each drop = 1 mg/L dissolved oxygen.
11. Clean-up by pouring the DO bottle solution into the plastic DO Waste Bottle. Next, use the clear solution in the square mixing bottle to rinse the DO bottle and test tube. Add the rinse to the waste bottle. If crystals remain in the DO bottle, rinse with a full square-mixing bottle of river water, swirl and empty into the waste bottle. If crystals still remain, repeat the rinse process.
Chemistry Theory for Dissolved Oxygen Kits
Higher-level science classes will be interested in the balanced chemical equations for each step of the dissolved oxygen test. The reactions for the Modified Winkler Method or Iodometric Method are described below.
Foil Packets #1 and #2
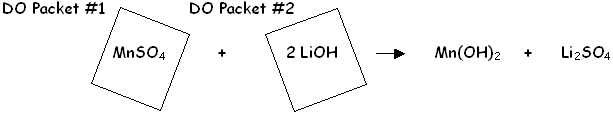
To start the Dissolved Oxygen (DO) Test, the reagents from the two foil packets are added into the river sample. This step combines manganese (II) sulfate from DO Packet #1 with lithium hydroxide base contained in DO Packet #2. Manganese (II) hydroxide is temporarily produced along with lithium sulfate.
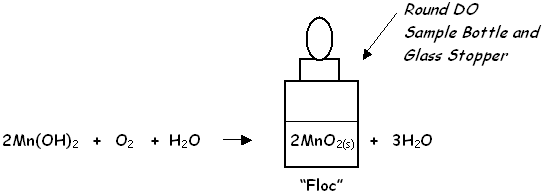
Dissolved oxygen in the river water then reacts immediately with the manganese (II) hydroxide to produce an orange manganese (II) oxide floc that gradually settles to the bottom of the sample bottle.
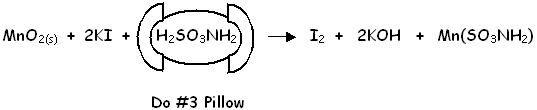
Sulfamic Acid Pillow #3
The orange manganese (II) oxide floc reacts with the potassium iodide from Reagent #2 and the sulfamic acid now added by Powder Pillow #3. This releases free iodine with a brownish colour. The dissolved oxygen is now "fixed", further air bubbles are not a problem and the final titration step can be delayed up to 8 hours. The focus for remaining procedures is now the iodine, not the oxygen.
Titration Solution
With the final titration step, the focus is on the iodine equivalent rather than on the original molecular oxygen. Each drop of titrant added to the iodine indicates that a greater amount of dissolved oxygen was present in the original river water. The Sodium Thiosulfate Solution is titrated drop-by-drop, reducing the iodine back to its ionic form and changing the colour from yellow-brown to clear.
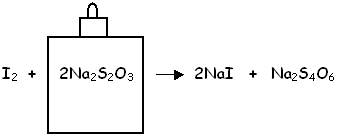
Interpreting Dissolved Oxygen Test Results
Measuring dissolved oxygen is one of the most important, if not the most important, tests of water quality for aquatic life. High levels of dissolved oxygen can indicate good water quality and a healthy ecosystem; lower levels can be an indication of pollution and environmental stress.
A Guide for Interpreting Dissolved Oxygen Concentrations
|
Dissolved Oxygen |
mg/L |
River Water Quality |
River Ecosystem |
|
High |
7 - 11 |
Excellent |
Healthy |
|
Medium |
4 - 7 |
Good |
Borderline Healthy |
|
Low |
2 - 4 |
Poor |
Unhealthy |
|
Very Low |
0 - 2 |
Very Poor |
Won't support life |
Interpreting Dissolved Oxygen Data
There are many slightly different interpretations of dissolved oxygen (DO) concentrations, but the trend is clear. Within the possible range of 1-14 mg/L...
- low concentrations indicate poor water quality and unhealthy ecosystems
- high concentrations indicate good water quality and healthy ecosystems.
Here is a selection of interpretations for dissolved oxygen results:
- The Global Water Sampling Project in New Jersey states that a dissolved oxygen level of 9-10 mg/L is considered very good; at levels of 4 mg/L or less, some fish and macroinvertebrate populations begin to decline.
- The Hach Chemical Company suggests that a dissolved oxygen content of 4-5 mg/L is considered borderline for aquatic life over extended periods of time. Sportfish populations require 8-14 mg/L and good fishing waters generally average 9 mg/L. At less than 3.0 mg/L, bottom-feeding fish (suckers) will die. While northern pike require at least 6.0 mg/L in the summer, these fish can get by with as little as 3.1 mg/L in the winter.
- The 1999 Canadian Water Quality Guidelines (CWQG) for the Protection of Aquatic Life suggest that total oxygen concentrations for freshwater should be 5.5-9.5 mg/L.
- According to "Stream Analysis and Fish Habitat Design", sportfish have differing requirements for dissolved oxygen at different temperatures:
|
Fish Species |
Temperature°C |
Minimal Dissolved Oxygen mg/L |
Optimal Dissolved Oxygen mg/L |
|
Brown trout |
colder than 15 |
at least 3 |
more than 7 |
|
Pike |
warmer than 15 |
1 |
|
|
Rainbow trout |
15 |
less than 3 is lethal |
7 |
- "Save Our Streams" states that trout need at least 6 mg/L at all times to function normally. At less than 3 mg/L, the water is considered oxygen poor.
- The FEESA "Aquatic Invertebrate Monitoring Program" states that 4-5 mg/L is the minimum value necessary to support aquatic life.
pH Test Kit Instructions
WARNING: The chemicals in this kit may be hazardous to the health and safety of the user if inappropriately handled. Please read all warnings carefully before performing the test and use appropriate safety equipment.
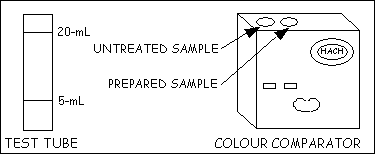
1. Place the test kit on the ground in a safe, dry place.
2. Fill the two glass test tubes up to the 5-ml mark with river water.
3. Wear goggles and gloves. Add four drops of Phenol Red Indicator Solution to one test tube and swirl to mix. This is the prepared sample.
4. Insert the test tube with the prepared sample solution into the inside hole on top of the black colour comparator box.
5. Insert the test tube with the untreated sample water into the outside hole on top of the black colour comparator.
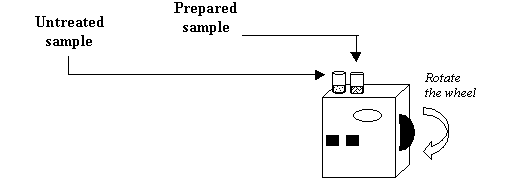
6. Hold the colour comparator up to the sky or sun and look through the two openings in the front. Rotate the colour wheel until a colour match is obtained. Read the number on the colour wheel scale.
7. Have another person try matching the colours and then read the number scale. Agree on the best value. Record the result on the data sheet.
8. Clean-up by pouring the colored prepared solution into the pH Waste Bottle. Use the clear untreated sample water to rinse the prepared test tube and add this to the waste bottle.
A Guide for Interpreting pH Levels
|
Range |
pH |
River Water Quality |
River Ecosystem |
|
High |
8.5 - 14 |
Poor |
Unhealthy |
|
Medium |
6.5 - 8.5 |
Good |
Healthy |
|
Low |
1 - 6.5 |
Good |
Unhealthy |
The pH is used to measure the relative acidity of solutions such as water.
Solution with a pH greater than 7.0 is considered to be basic or alkaline. The greater the pH, the greater the alkalinity.
Distilled water has a pH of 7.0. This is considered neutral.
Solutions with a pH level less than 7.0 are considered to be acidic. The lower the pH, the more acidic the solution.
A pH range of 6.5 - 8.5 is often considered safe for fish and aquatic invertebrates.
Nitrogen Test Kit Instructions
A Guide for Interpreting Ammonia Levels
|
Ammonia Nitrogen Concentrations |
mg/L |
River Water Quality |
River Ecosystem |
|
Low |
1.0 or less |
Excellent |
Healthy |
|
Medium |
1.0 - 3.0 |
Good |
Borderline Healthy |
|
High |
3.0 - 5.0 |
Fair |
Unhealthy |
|
Extreme |
5.0 or greater |
Poor |
Very Unhealthy |
Adapted from "Field Manual for Global Low-Cost Water Quality Monitoring"
The Canadian Water Quality Guidelines (CWQG) for the Protection of Aquatic Life suggest that total ammonia concentrations for freshwater should be no more than 1.3-2.2 mg/L.Phosphorus
Simply put, phosphorus makes life on earth possible. It is an important plant fertilizer and animals require it for:
- bones
- teeth
- blood plasma
- cell chemistry
- genetic material
Phosphorus is normally present in rivers at low concentrations. Too much dissolved phosphorus can set off a chain of undesirable events:
- Extra phosphorus increases the growth of aquatic plants and algae.
- Bacteria eventually decompose the dead plant material.
- Decomposition removes dissolved oxygen from the water.
- Fish die if dissolved oxygen drops to critical levels.
High levels of dissolved phosphorus are an indicator of pollution. Pre-packaged chemistry kits can be used to measure the concentration of dissolved phosphorus. Excessive phosphorus can enter a river from two major sources:
- sewage effluent from towns and cities
- storm water that drains from streets and agricultural land
An understanding of basic phosphorus science can help interpret data collected from local rivers.
Phosphorus Background Information
Phosphorus occurs most commonly bonded with oxygen atoms to form a phosphate. Phosphates are essential chemicals found naturally in all living organisms, soil and water. For example, bones contain calcium phosphate.
Surface water supports the growth of microscopic floating organisms called plankton. One type of plankton is algae. The growth of algae and aquatic plants requires phosphates.
Humans use phosphates in dental cements, water softeners, detergents, rust proofing and processed foods. Phosphoric acid is used in cola soft drinks. Dicalcium phosphate is a food supplement for cattle. Calcium phosphate is an ingredient in plant fertilizers.
What is Phosphorus
Phosphorus is a chemical element identified with the symbol (P). It was discovered in 1669 by the German chemist Henning Brand who prepared it from urine samples.
Phosphorus is the 11th most abundant element in the earth's crust and the second most abundant mineral in the human body. Foods containing high amounts of phosphorus include dairy products, eggs, fish, dried fruit, meat, garlic, nuts and whole grains. North Americans ingest about 1500 milligrams of phosphorus daily, which is almost twice the recommended allowance.Phosphorus combines readily with oxygen to form oxides, phosphates and a mineral called apatite. The phosphorus required by living things is combined with oxygen and called a phosphate (PO4-3). Phosphates are found dissolved in water (inorganic phosphates) and within living tissue (organic phosphates).
Types of Phosphate
Phosphorus occurs most commonly bonded with oxygen atoms to form a phosphate ion (PO43-). However, there are many other forms of phosphate:
- Inorganic Phosphates
Inorganic phosphates are also called mono, reactive, dissolved, soluble or orthophosphates. Inorganic phosphates are not a part of living tissues and they are not carbon-based.
calcium phosphate Ca3(PO4)2
is an inorganic molecule found in bones
Animals require inorganic phosphorus for bones, teeth, blood plasma and a regular heartbeat. Inorganic phosphorus is absorbed by plants from water or soil.
- Organic Phosphates
Organic phosphates are a part of plants and animals, their wastes or their decomposing remains. Organic phosphates consist of a phosphate ion bonded with a carbon-based molecule.
e.g. adenosine triphosphate (ATP)
is an organic phosphate built on a 5-carbon ribose sugar C5H10O5
Organic phosphates are found in cell membranes, genetic molecules (DNA, RNA) and energy storing molecules (ADP and ATP).
- Polyphosphates
Human-made phosphates are complex inorganic compounds called condensed, meta or polyphosphates. These types of phosphates are used in laundry detergents, commercial cleaners, water treatment and industrial boilers.
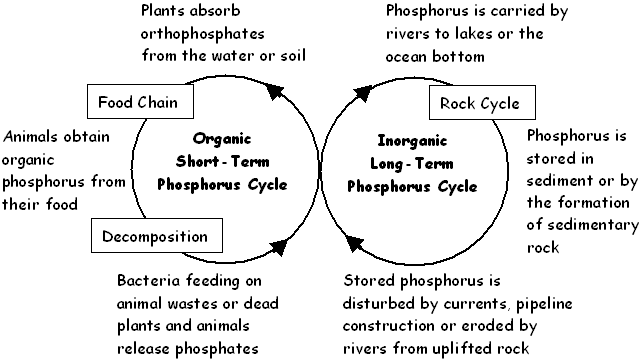
How Is Phosphorus Cycled Through Ecosystems?
There are two main processes occurring in all ecosystems - energy flow and material cycling. Phosphorus is cycled between living organisms and the earth's crust as energy flows through a food web.
Phosphorus in Living Organisms
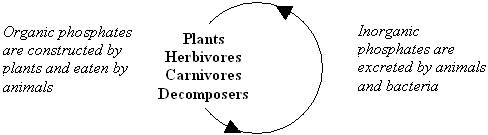
In aquatic ecosystems, the short-term cycling of phosphorus is through the food web of living organisms.
- Plants absorb inorganic phosphates through their roots and convert them into organic phosphates.
- Animals obtain their phosphorus by eating plants or other animals. Animals excrete inorganic phosphorus in urine.
- Bacteria decompose dead plants and animals and then release inorganic phosphorus back into the environment to continue the cycle.
Phosphorus in the Earth's Crust
If left undisturbed for millions of years, bottom sediments transform into phosphorus-containing rock.During the long-term cycling of phosphorus in the Earth's crust, phosphorus leaches out of soil and weathers out of rock. This inorganic phosphorus flows downstream and eventually accumulates at the bottom of rivers, lakes and oceans.
"Stored" phosphorus may return again to the surface during the uplifting of mountains, during the mining of potash or when bottom sediments are disturbed.
The phosphorus cycle starts again as water erodes the uplifted phosphorus rock.
The Phosphorus Cycle
Phosphorus moves between plants, animals, bacteria, rock, soil and water. This is called a biogeochemical cycle. Three natural processes contribute to this cycling of phosphorus - food webs, decomposition and the rock cycle.